Clinical Research
Clinical Research in Homoeopathy helps in generating, validating and consolidating scientific evidences (in terms of safety, efficacy and effectiveness) of homoeopathic medications, procedures and treatment regimes. These researches may be useful in in prevention, treatment of various diseases, decision making for stake holders and thus help in improving clinical care. The aim is to carry out evidence-based trials based on modern scientific parameters (double blinding; objective assessment criteria, statistical analysis, etc.) without conflicting with the doctrines of Homoeopathy. More emphasis is laid upon the clinical evaluation of homoeopathic medicines in disease conditions of national health importance, where no curative treatment is available in conventional medicine; endemic diseases in certain parts of the country and the so-called surgical diseases.
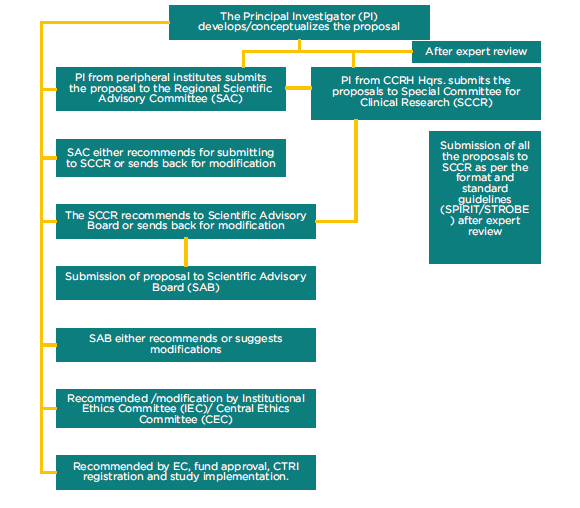
Being one of the major activities of the Council, Clinical research has traversed a path from prospective observational studies to gold standard randomized controlled studies. Studies in various diseased conditions are taken up from time to time as per the guidance of Scientific Advisory Committee (SAC). Earlier, multi-centric clinical studies were conducted to ascertain therapeutic utility of a smaller group of medicines on the protocols developed in consultation with the experts in respective fields from All India Institute of Medical Sciences (AIIMS), Indian Council of Medical Research (ICMR), National Institute of Communicable Diseases (NICD), National AIDS Control Organization (NACO), eminent homeopathic Educators and Researchers. Presently as per the need of the hour, randomized controlled trials (RCT) are being conducted to establish the effectiveness of homoeopathic treatment. The results of these studies are published time to time in the National, International peer reviewed journals of importance.
Till date Council has conducted 238 studies on various diseases, out of which 195 studies are concluded (154 observational studies and 41 randomized clinical trials). Currently 5 studies are ongoing. The salient achievements in clinical studies have been in HIV/AIDS, Gastroenteritis, Chronic Sinusitis, Influenza like illness, Benign prostatic hyperplasia, acute haemorrhoids, cervical spondylosis pain management, urolithiasis , Acute Rhinitis in children, Acute encephalitis syndrome, Covid-19, Acute otitis media, hypertension, thrombocytopenia due to dengue.
CLINICAL RESEARCH STUDIES
i) Concluded Studies : Download
ii)Ongoing Studies : Download
iii)Clinical Research Publication : Download






.png)

.png)