Drug Standardisation
Success in homoeopathic prescribing is based upon the purity and quality of raw drugs & finished products. A sub-standard drug will not produce desired results in a sick individual. Drug standardisation encompasses a comprehensive evaluation of the homoeopathic drugs in respect of their pharmacognostical, physico-chemical and pharmacological profiles in order to study the various qualitative and quantitative characteristics of drugs. The pharmacognostical studies of raw drug plant material include study of the gross morphology of the raw drug, its macro and microscopical characteristics and after suitable processing enumeration of characteristic structures of cells, tissues and organs under the microscope and determining their essential bio statistical dimensions. The physico-chemical parameters of the raw drug and prepared mother tincture include moisture content, ash value, extractive value, presence of active constituents in raw drug and organoleptic characteristics, carrying out specific tests and TLC, UV spectrophotometry of the mother tincture. These can be used as a bench mark standard against any commercial sample to be compared with in future or as reference whenever there is a necessity. The pharmacological spectrum of a drug is ascertained through experimental trials on laboratory animals under standard laboratory conditions which include preliminary estimation of dosage, evaluation of efficacy and safety and also the mode of action of homoeopathic drugs.
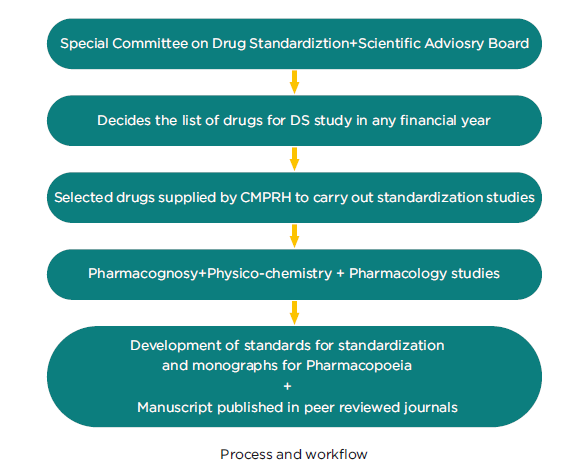
The council has undertaken pharmacognostical studies on 368 drugs, physico-chemical studies on 362 & pharmacological studies on 151 drugs (as in March 2016).149 drugs have been studied in all the three aspects. The standards of the drugs worked out by the Council are definite gains and are important for preparation of quality homoeopathic medicines.
These findings have been published in research journals; monographs and books of the Council:
- Standardisation of Homoeopathic Drugs Vol. I- IV
- Identification of Homoeopathic Drugs of Plant Origin
- Pharmacological action of Homoeopathic drugs and
- Vernacular names of plant drug in Homoeopathic Pharmacopoeia of India : which contains data of 548 plant drugs incorporated in Homoeopathic Pharmacopoeia of India (HPI) Volumes I-IX in various languages to identify them locally. This compilation has been made as an effort to improve the Homoeopathic Pharmacopoeia of India bringing it at par with other world class pharmacopoeias.
At present, pharmacognostical & physico-chemical studies are being undertaken at two centres of the council:
1. Dr D. P. Rastogi Central Research Institute (H), Noida
2. Drug Standardisation Unit (H), Hyderabad (A.P.)
Laboratories involved in Drug Standardization:

Laboratory at DDPRCRI(H), Noida

Zebra Fish lab, DDPRCRI(H), Noida

Zebra Fish lab, DDPRCRI(H), Noida

Animal House, DACRRI(H), Kolkata
The pharmacological studies under Drug Standardization programme have been suspended since July 1999 due to certain conditions imposed by the Ministry of Social Justice and Empowerment for conducting animal experimentations.
List of Drugs Studies under Drug Standardization:Download
Concluded:Download






.png)

.png)