Drug Proving
Drug Proving also termed as Homoeopathic Pathogenetic Trial (HPT) is a process in which drug substances are put into trial on healthy human volunteers and their pathogenetic effects are observed, noted and compiled as the first step to introduce the drug in the Homoeopathic Materia Medica. Proving of a drug substance is a unique process in Homoeopathy. Unlike conventional medicine where animal experimentation forms the basis of evaluation of drug pathogenesis, homoeopathic medicines are proved on healthy human volunteers, including controls, from both sexes and age group between 18-60 years. The Central Council for Research in Homoeopathy since its inception in 1978 has adopted the Drug Proving Research Program as one of its primary research areas where Council has focused on proving of indigenous drugs and fragmentarily proved drugs.
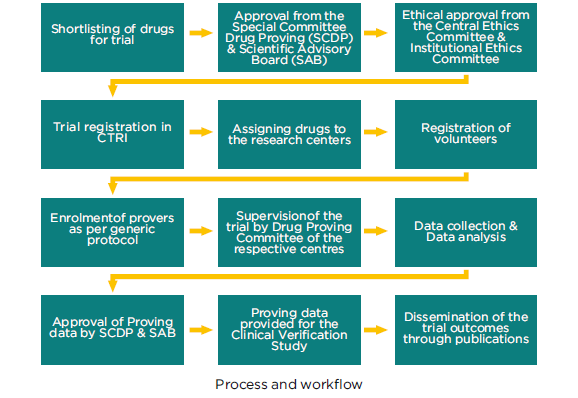
Double-blind, randomized and multi-centric trials are being conducted at the following centres:
1. Dr. D. P. Rastogi Central Research Institute (H), Noida (Uttar Pradesh)
2. Central Research Institute (H), Kottayam (Kerala)
3. Regional Research Institute (H), Kolkata (West Bengal)
4. Homoeopathic Drug Research Institute, Lucknow (Uttar Pradesh)
5. Regional Research Institute (H), Navi Mumbai (Maharashtra)
6. Regional Research Institute (H), Gudivada (Andhra Pradesh)
7. Drug Proving Unit, Bhubaneswar [Extension Unit of Regional Research Institute (H), Puri, Odhisa]
8. Regional Research Institute (H), Jaipur (Rajasthan)
DRUG PROVING PROTOCOL
i) Generic Drug Proving Protocol : Download ![]() 903KB
903KB
ii) Drug Proving Protocol for re-proving of the drugs : Download ![]() 906KB
906KB
DRUG PROVING STUDIES
i) Concluded Studies : Download ![]() 240KB
240KB
ii) Ongoing Studies : Download ![]() 66KB
66KB
Homoeopathic Pathogenic Trial (Drug Proving Research) Portal






.png)

.png)